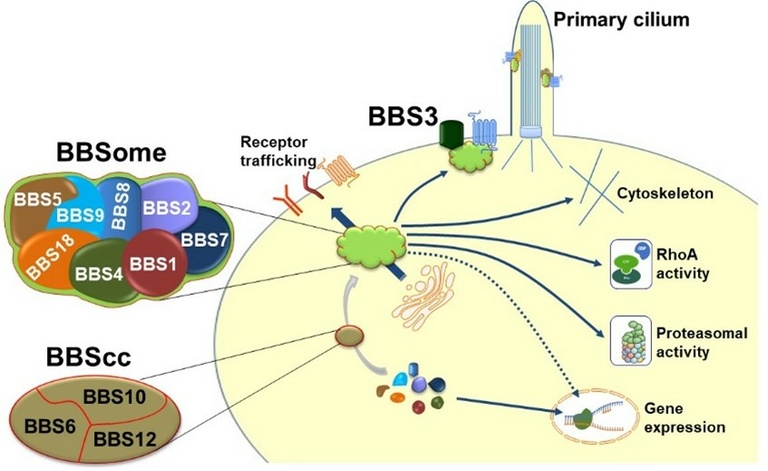
Ongoing studies in the laboratory focuses on dissecting the cellular and physiological relevance of a protein complex termed BBSome which is composed of several Bardet-Biedl Syndrome (BBS) proteins. Humans carrying mutations in the genes encoding BBS proteins display many phenotypes including obesity and type 2 diabetes. Since these phenotypes are common in the general population our goal is to understand the significance of the BBSome for regulation of energy and glucose metabolism. We discovered that the BBSome is critically involved in the regulation of energy balance and glucose metabolism by mediating the trafficking of key metabolic receptors (e.g., leptin receptor and inulin receptor) to the cell membrane. These findings challenged the dogma that the BBSome is solely involved in the regulation of the function of cilia, hairlike cellular projections found in virtually all organs and cell types of the mammalian body. Instead, our work demonstrates that the BBSome is implicated in the control of cellular processes beyond the cilium that impact metabolic health. In addition, we uncovered important functions of the BBSome in various cell types and tissues including those related to cardiovascular health and disease.
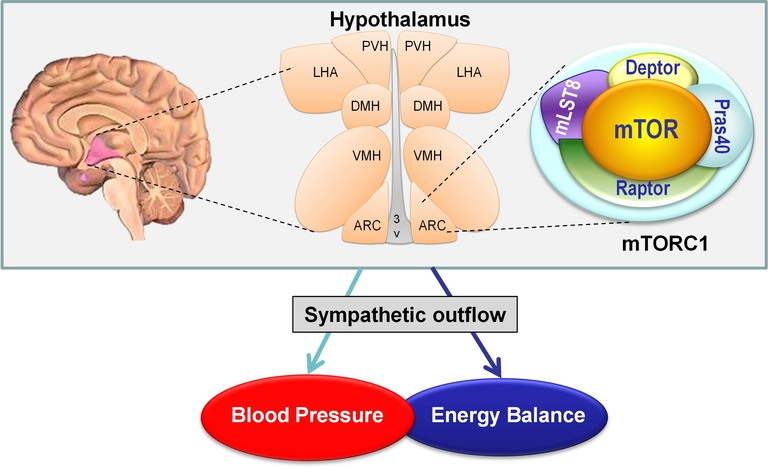
Another major area of interest of the laboratory relate to the neuronal mechanisms and brain regions involved in the regulation of regional sympathetic nerve activity and attendant metabolic and cardiovascular responses. We have created a body of work that established the extreme selectivity of the neurocircuit involved in the regulation of regional sympathetic outflow involved in the control of energy homeostasis and blood pressure. We defined the exact role of distinct neuronal populations, such as hypothalamic neurons expressing proopiomelanocortin neurons and agouti-related protein, in the control of sympathetic outflow to various peripheral tissues impacting different physiological processes including blood pressure, hepatic function and adipose tissue. Moreover, we identified hypothalamic mTORC1 (mechanistic target of rapamycin complex 1) signaling as a critical substrate for the neuronal control of sympathetic nerve activity and blood pressure. We extended this work to implicate mTORC1 signaling in mediating the regulation of vascular function. In addition to mTORC1, we have uncovered the neuronal actions of several new proteins and hormones including FGF21, RAMP1, Bmp8b, amylin, thyroid hormones and estradiol as critical regulators of sympathetic traffic with important implications on metabolic and cardiovascular homeostasis.